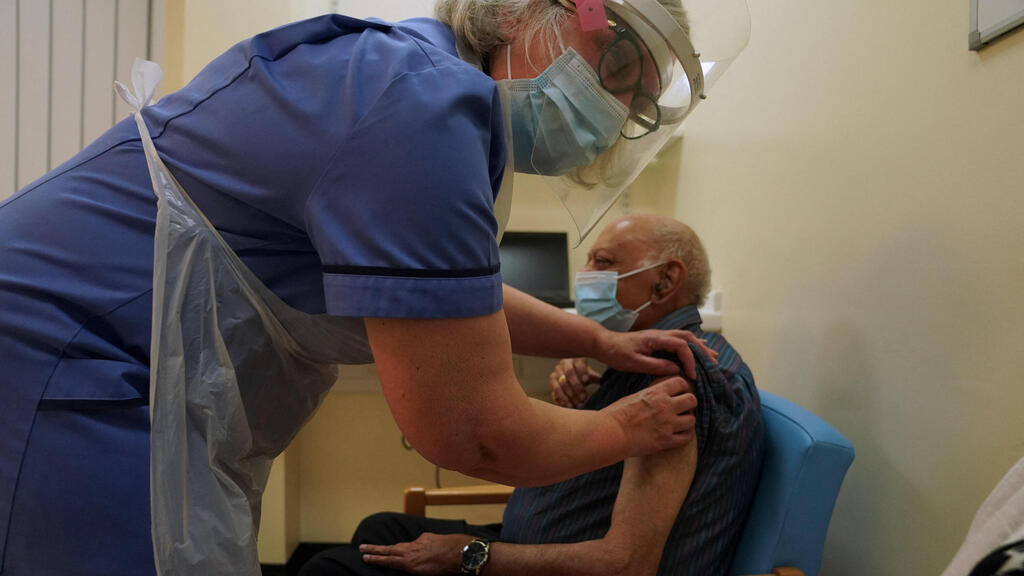
UK regulators say people who have a "significant history" of allergic reactions shouldn't receive the new Pfizer-BioNTech coronavirus vaccine, while they investigate two adverse reactions that occurred on the first day of the country's mass vaccination program.
The U.K. began vaccinating elderly people and medical workers with a vaccine developed by American drugmaker Pfizer and Germany's BioNTech on Tuesday, the world's first rollout of the vaccine.
Professor Stephen Powis, national medical director for the National Health Service in England, said health authorities were acting on a recommendation from the Medical and Healthcare Products Regulatory Agency, the nation's medicines regulator.
"As is common with new vaccines, the MHRA has advised, on a precautionary basis, that people with a significant history of allergic reactions do not receive this vaccination after two people with a history of significant allergic reactions responded adversely yesterday," Powis said in a statement. "Both are recovering well."
Dr. June Raine, head of the U.K.'s medical regulatory agency, reported those reactions as she testified Wednesday to a Parliamentary committee.
"We're looking at two case reports of allergic reactions," she said. "We know from the very extensive clinical trials that this wasn't a feature. But If we need to strengthen our advice, now that we have had this experience with the vulnerable populations, the groups who have been selected as a priority, we get that advice to the field immediately," she said.
The findings were revealed during a general discussion of how the agency will continue to monitor people who receive the Pfizer vaccine, which was authorized for emergency use last week.