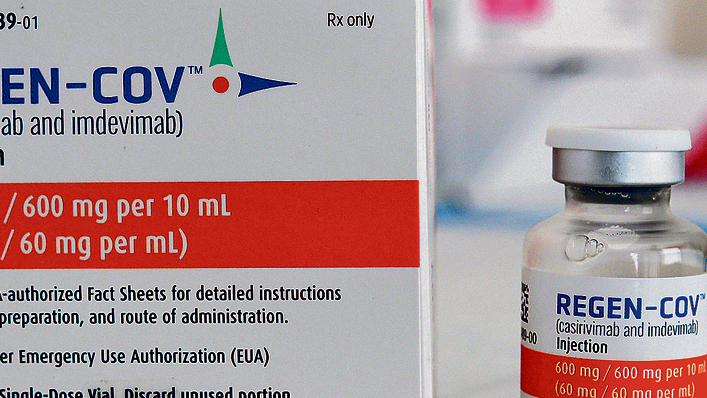
A potentially life-saving treatment is allegedly being withheld by health officials from Israelis suffering from COVID-19 despite the drug having an FDA approval and a proven rate of success in the United States.
Regeneron, is a cocktail of two monoclonal antibodies that was designed specifically to block infectivity of SARS-CoV-2, the virus that causes COVID-19, and is meant to be used by patients that have not been hospitalized, over the age of 65 who are at risk for developing a serious illness. before one develops.
Dr. Oren Kobiler, a virologist from the Tel Aviv University said the treatment can be administered in a shot or by an intervenes drip in the first days of infection. "The aim is to prevent severe complications from developing."
Former U.S. president Donald Trump allegedly received the drug during his COVID illness.
The Health Ministry has already purchased thousands of doses of the drug but has yet to release it for mass use by HMOs who treat coronavirus patients at home.
"My father began feeling ill a couple of weeks ago," Moti Leibowitz, the son of Yehuda who died earlier this week, said. "We tried to get him treated by Regeneron, but our HMO said they don't have the drug and even said they knew nothing about it," he said.
"You have to know where to go to get it and we found one of the aid organizations that could provide the drug, but my father refused to go to hospital to receive it. If he had been able to get it while at home, he would not have died," Leibowitz said.
2 View gallery

A coronavirus ward at the Barzilai Medical Center in Ashkelon earlier this month
(Photo: AP)
Senior health officials say the ministry is bogged down by bureaucratic infighting and the public is paying the price. "It is possible and necessary to purchase a large quantity of Regeneron," they said.
"The drug must be administered in the early stages of the disease to be most effective, and not when a patient's condition deteriorates and requires hospitalization," Professor Galia Rahav Head of the Infectious Disease Department at the Sheba Medical Center, explained.
"HMO's must be responsible for administering it to patients who are treated in the community," she said.
But Dr. Erez Carmon who heads the coronavirus response at one of the HMO's disagrees.
"Some drugs must be administered in a hospital setting," he said. "HMOs do not determine what drugs are available. That is the Health Ministry's job.
The Health Ministry said in response to a query from Ynet that the existing supply of Regeneron has been directed for use in care homes for senior citizens "but further use of the medication will be considered," the ministry said.