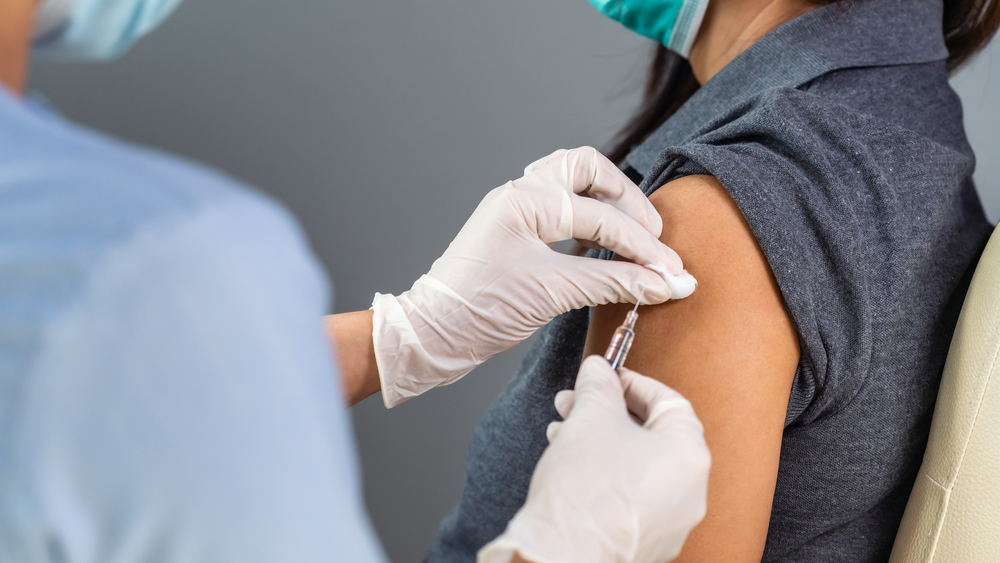
The Israel Institute for Biological Research announced on Monday that it had been authorized by the Health Ministry to begin the second phase of human trials for its coronavirus vaccine candidate.
In a statement, the institute said the ministry gave its approval to continue to the second phase of human trials after internal and external expert panels examined the results of the first stage of trials, which ended successfully and during which volunteers did not display side effects.
3 View gallery
The coronavirus vaccine produced by the Israel Institute for Biological Research
(Photo: Defense Ministry spokesperson)
Phase two will include 1,000 volunteers over the age of 18.
According to the statement, phase two aims to ensure the safety of the vaccine and determine the most effective dosage.
This phase will be run over a number of months. The third stage of human trials will test the vaccine on 30,000 volunteers in Israel and abroad.
The trial will begin at first at Jerusalem's Hadassah Medical Center and Sheba Medical Center near Tel Aviv initially and will later expand to other hospitals around the country.
3 View gallery


A coronavirus vaccine developed by the Israel Institute for Biological Research
(Photo: Defense Ministry spokesperson)
In remarks made on Monday, Defense Minister Benny Gantz called the Biological Research Institute's researchers "the finest in the field."
"They have taken upon themselves the most critical mission of saving lives," Gantz said. "I see great importance in the continued development of an Israeli vaccine that will serve the Israeli population for years to come."
Hospitals participating in the clinical trial stand to receive up to NIS 4.5 million ($1.35 million), Ynet has learned. Volunteers will be paid up to 6,500 shekels depending on the number of vaccine doses they receive.
The state will transfer NIS 20,000 to hospitals for each volunteer who agrees to take one dose and NIS 30,000 for each volunteer who agrees to take two doses. Further grants may be given for their participation and to cover staff costs.
Ynet has also learned the Israeli institute will not directly carry out the trials, but the U.S.-based clinical research company IQVIA which operates a branch in Israel.