Teva Pharmaceutical Industries is betting up to $500 million on a new antibody treatment it says could become a major future growth driver, targeting autoimmune diseases with limited treatment options, including vitiligo and celiac disease.
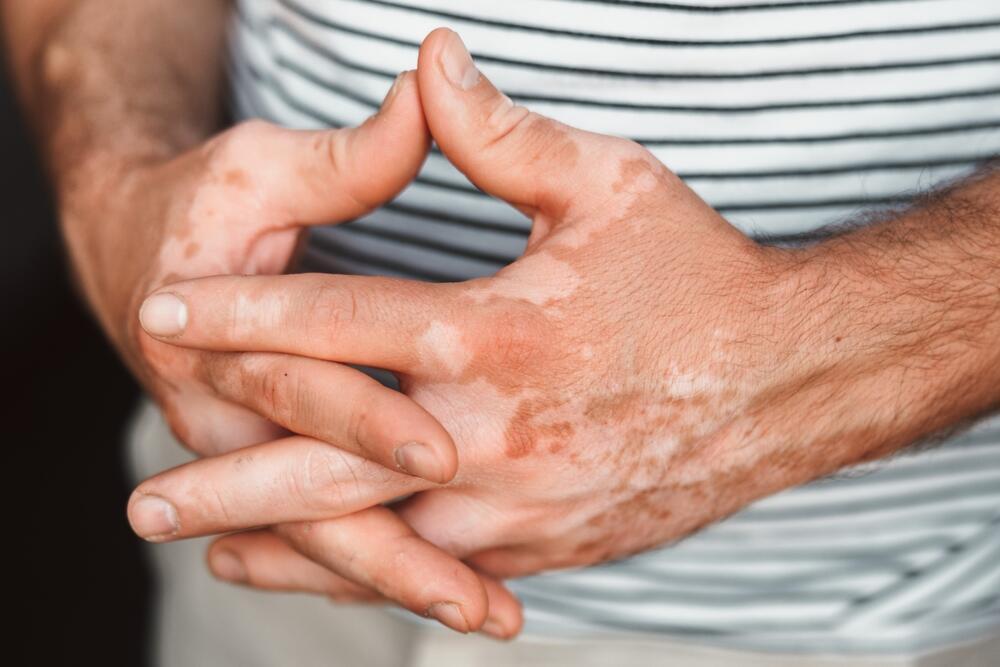
The product at the center of the deal, known as TEV-408, is not aimed at a widespread or deadly illness but at vitiligo, a chronic autoimmune skin disease that affects a small percentage of the population and has a deep impact on quality of life. For most patients, treatment options remain limited.
Teva estimates the vitiligo therapy could generate annual sales of about $1 billion if successful. The company is also developing TEV-408 as a treatment for celiac disease, an autoimmune disorder triggered by gluten that damages the small intestine. The drug is currently in Phase 2 clinical trials for celiac disease, with analysts estimating its sales potential at about $1.5 billion annually, higher than that projected for vitiligo.
Dr. Shany Sherman, deputy director of the dermatology department at Beilinson Hospital, part of Clalit Health Services, described the development as unprecedented.
“This is a drug that could fundamentally change how vitiligo is managed,” Sherman said. “Until now, treatments have focused on suppressing the disease or calming it temporarily. This approach is aimed at curing the disease itself, not just controlling it.”
According to Sherman, the antibody targets a population of immune memory cells that remain dormant in the skin and can reactivate after periods of remission, triggering renewed disease activity. By eliminating these cells, the treatment aims to shift care from temporary suppression to long-term remission. She cautioned that the drug is still in development and faces additional trial stages, noting that other vitiligo treatments, including oral medications, are further along in development and may seek U.S. approval in the near future.
Vitiligo is an autoimmune condition in which the immune system destroys melanocytes, the cells responsible for skin pigmentation, resulting in white or light patches on the skin. It affects an estimated 0.5% to 2% of the global population and can be accompanied by psychological and social challenges, including depression and social isolation. Currently, only one topical medication is approved, and its use is limited to up to 10% of body surface area, leaving many patients without adequate treatment. The disease became widely known after pop star Michael Jackson disclosed in 1993 that he had vitiligo.
TEV-408 is a systemic antibody designed to neutralize the protein IL-15, a key cytokine involved in autoimmune processes. Teva said laboratory data show the antibody binds effectively to IL-15 and has a long duration of action. The drug is intended for self-administration through subcutaneous injection, targeting the underlying immune mechanisms rather than surface symptoms.
IL-15 is involved in multiple autoimmune diseases, not only vitiligo. Teva said interim data from a Phase 1b trial suggest blocking IL-15 could be relevant across a broader range of immune-mediated conditions.
In celiac disease, IL-15 plays a central role in driving the immune response to gluten exposure. Celiac affects an estimated 1% to 3% of the global population, though many cases remain undiagnosed. Symptoms range from digestive problems and nutrient deficiencies to anemia, growth delays in children or no symptoms at all. The only proven treatment is a strict, lifelong gluten-free diet.
TEV-408 is currently being tested in a Phase 2a trial for celiac disease. In May 2025, the U.S. Food and Drug Administration granted the drug Fast Track status for the indication, a designation intended to speed development of treatments for conditions with unmet medical needs.
Teva President and CEO Richard Francis has said the program reflects the company’s strategy to build a pipeline focused on innovative biologic therapies with the potential to address serious unmet needs and generate long-term growth.