U.S. drugmaker Moderna Inc said on Monday that Israel's health authorities had authorized its COVID-19 vaccine, marking the vaccine’s third regulatory authorization and the first outside North America.
Moderna announced in November that its version of a coronavirus vaccine proved to be 94.5% efficient in clinical trials.
“Health Ministry of Israel has secured 6 million doses and first deliveries (are) expected to begin in January,” Moderna said in a statement.
Moderna has received authorization for its COVID-19 vaccine in the United States and Canada and additional authorizations are currently under review in the European Union, Singapore, Switzerland and the United Kingdom.
The company requires vaccines to be shipped and stored in -15 degrees centigrade allowing storage in regular freezers, unlike Pfizer's required -70 degrees.
After thawing, a vial of the Pfizer vaccine must be used within five days while Moderna’s is stable at fridge temperature for 30 days and at room temperature for 12 hours.
Israel has begun to vaccinate its population at one of the quickest rates in the world, and it aims to reach all vulnerable citizens by late January. Authorities started vaccinations on Dec. 19 using the vaccine developed by Pfizer and BioNTech.
2 View gallery
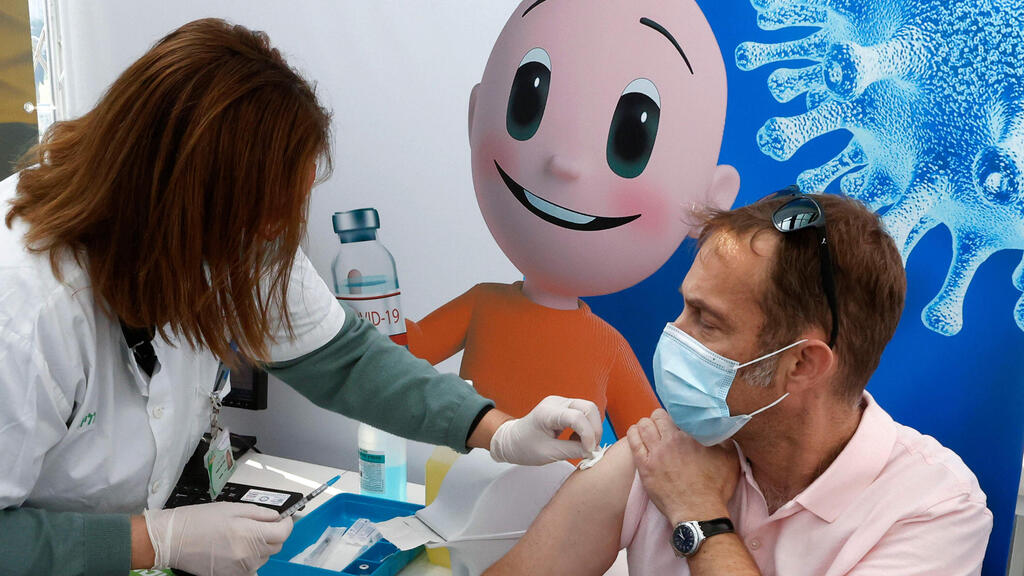

A man receives his first dose of the coronavirus vaccine on Monday in Tel Aviv
(Photo: AFP)
The Bank of Israel said on Monday it expects the economy to rebound quickly in 2021 if the country’s fast start to vaccinating people against COVID-19 is maintained.
Moderna said separately on Monday it would produce at least 600 million doses of its COVID-19 vaccine in 2021, up by 100 million doses from its previous forecast. The company was working to invest and hire in order to deliver up to 1 billion doses this year.
Moderna has so far supplied about 18 million doses to the U.S. government as part of a deal for 200 million doses. It has also signed a deal with the Canadian government for 40 million doses.
First published: 06:26, 01.05.21