Engineering proteins in the 'Protein Repair Garageffect millions worldwide, particularly children, with an estimated 263 million cases reported in 2023 across 83 countries.
A significant challenge in combating malaria is the Plasmodium vivax parasite, which can enter a dormant state within the liver, evading detection and leading to recurrent infections.
In a groundbreaking study published in the Journal of Biological Chemistry, researchers at the Weizmann Institute of Science significantly enhanced the stability and durability of a protein used in malaria diagnostics. This innovation enables the detection of the parasite even in its dormant state, potentially making diagnostic tools more affordable, reliable and accessible in regions heavily burdened by malaria.
Understanding Malaria and the challenges of eradication
Malaria is a severe disease transmitted by the bite of an infected female Anopheles mosquito. While once prevalent in areas like Israel, the disease remains a major health threat in regions such as Africa, the Amazon, Southeast Asia and India. Approximately three billion live in malaria-endemic areas.
“Malaria is caused by five species of parasites from the Plasmodium family, transmitted through mosquito bites,” explains Prof. Sarel Fleishman, head of the Protein Design Laboratory at the Weizmann Institute of Science. “The most prevalent and deadly species in Africa is Plasmodium falciparum. This single-celled parasite is responsible for approximately 600,000 deaths annually, most of which occur in children under the age of five. Survivors often endure severe symptoms.”
Outside Africa, the dominant species is Plasmodium vivax, the primary focus of recent research at the Weizmann Institute. This parasite is the second most lethal and is the leading cause of malaria in South America and Southeast Asia.
Efforts to eradicate malaria have included draining swamps, preventive treatments and widespread use of insecticides. While significant progress was made by 2015, with a notable reduction in cases, advancements have since stalled. The persistence of Plasmodium vivax plays a major role in this stagnation.
The parasite can remain dormant in the liver for unpredictable durations—sometimes months—without causing symptoms. During this latency period, patients often discontinue treatment, believing they have recovered. However, once reactivated, the parasite can cause a relapse, not only resulting in repeated illness for the host but also serving as a source of further infections.
“When an infected individual is bitten by an Anopheles mosquito, the parasite can be transmitted to another person, reigniting the chain of infection,” explains Prof. Fleishman. “This creates an ongoing cycle where previously treated patients continue to spread the disease, as the dormant parasite evades standard diagnostic tests. Currently, around 80% of malaria cases are due to such recurrences, making eradication incredibly challenging.”
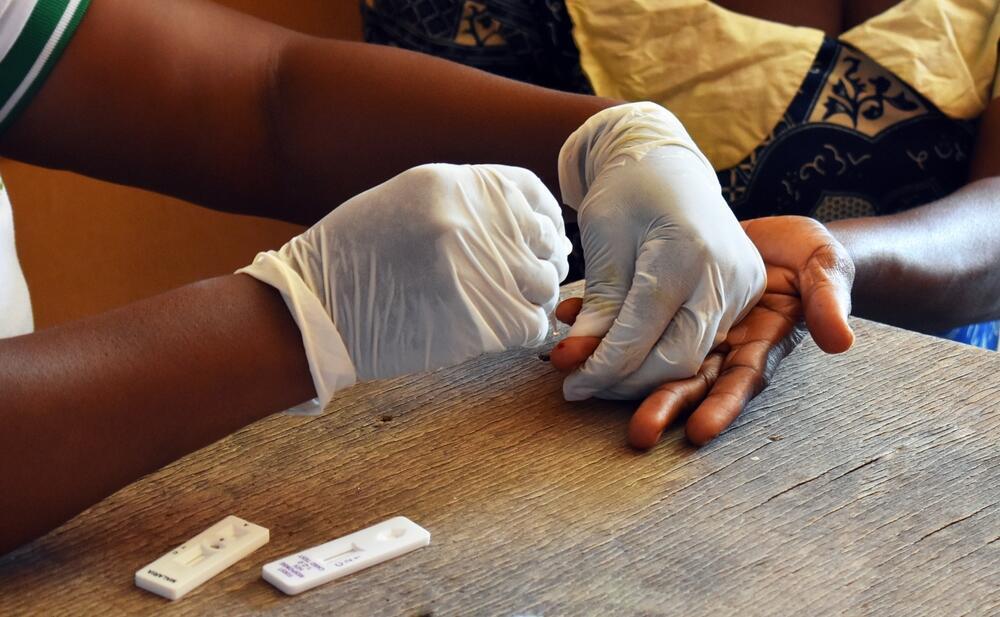
A novel diagnostic test with game-changing potential
Until recently, there were no effective tools to detect the dormant Plasmodium vivax parasite in the liver. However, five years ago, researchers at the WEHI Institute in Australia discovered that specific proteins produced by the parasite could be detected in the bloodstream. This breakthrough offered a new opportunity: the development of a simple blood test capable of identifying patients who appear to have recovered but still harbor the dormant parasite.
“This discovery enables the development of an antigen test—similar to home COVID-19 tests—that can detect the parasite’s presence by identifying antibodies in the blood,” notes Prof. Fleishman. “This has critical implications. Currently, drug treatments are halted as soon as symptoms subside. However, if a diagnostic test could confirm the parasite’s continued presence, treatment could be extended, preventing relapses and further transmission. Such a diagnostic tool has the potential to revolutionize global efforts to combat malaria and significantly aid in its eradication.”
However, the central protein used in the diagnostic panel presents a significant challenge: it is not sufficiently stable, making its production prohibitively expensive. Additionally, the scale of production needed to ensure global accessibility is immense—hundreds of millions, if not billions, of test units.
Another obstacle is the protein’s stability in extreme environmental conditions. Proteins are inherently sensitive molecules, and under harsh conditions—such as the high temperatures and humidity typical of malaria-endemic regions—they are prone to degradation, reducing the diagnostic tool’s effectiveness. Developing a stable, cost-effective version of the protein is thus a critical challenge in the fight against malaria.
Engineering proteins in the 'Protein Repair Garage'
Prof. Fleishman’s laboratory at the Weizmann Institute of Science is a global leader in protein engineering, yet improving the diagnostic protein presented a formidable challenge. Under the leadership of doctoral researcher Lucas Kraus, the team developed a novel computational approach to design three new variants of the protein. These variants were proven to be significantly more stable, durable and cost-effective to produce, while maintaining their diagnostic efficacy.
 Prof. Sarel FleishmanPhoto: Weizmann Institute
Prof. Sarel FleishmanPhoto: Weizmann Institute“Several years ago, we developed tools to enhance protein stability without compromising their function,” explains Prof. Fleishman. “We call these tools the ‘Protein Repair Garage.’ These methods have already been successfully used to develop a vaccine against Plasmodium falciparum, the dominant malaria parasite in Africa. However, this project posed a unique challenge: the protein being engineered is used to detect the presence of the malaria parasite in the body, and its primary role is to bind to nine distinct human antibodies.
Get the Ynetnews app on your smartphone: Google Play: https://bit.ly/4eJ37pE | Apple App Store: https://bit.ly/3ZL7iNv
“For the diagnostic test to be reliable, the protein must retain its ability to bind all nine antibodies. This requirement significantly restricted the types of structural changes we could make. It was like solving a puzzle with one hand tied behind your back. By combining artificial intelligence and advanced physical modeling, we were able to make precise modifications to the protein while preserving its functionality.”
The final protein variant contains 20 targeted mutations, significantly increasing its stability—up to temperatures nearing 60°C—without compromising its diagnostic capabilities. Moreover, this new version can be produced in quantities 15 times greater than the original, reducing production costs by more than tenfold. Tests conducted by research collaborators in Australia confirmed that the engineered protein retains its ability to bind all nine antibodies with the same effectiveness as the natural protein. This makes it an excellent candidate for improving malaria diagnostic tests.
From intelligence research to protein engineering
Prof. Fleishman’s journey into protein research began during his military service as an intelligence analyst, where he discovered a passion for tackling complex research problems. After completing his service, he pursued studies in the Adi Lautman Interdisciplinary Program at Tel Aviv University, focusing on molecular biology, chemistry, mathematics and philosophy. He later completed his master’s and doctoral studies in computational methods for predicting protein structures and functions. His postdoctoral research was conducted in the lab of Nobel laureate Prof. David Baker, who was awarded the Nobel Prize in Chemistry in 2022 for pioneering computational protein design.
During his postdoc, Prof. Fleishman developed the first computational method for designing proteins capable of binding specific molecular targets. This technique was used to create new proteins that inhibit various strains of the influenza virus, including the Spanish flu, which caused millions of deaths a century ago.
“The research on malaria diagnostic proteins is another milestone in this journey—a convergence of cutting-edge computational techniques and the urgent need to address global medical challenges,” says Prof. Fleishman. “Just as my lab has previously enhanced proteins for malaria vaccines in Africa, this development has the potential to transform the approach to diagnosing malaria.”