The Health Ministry said Wednesday it will purchase Regeneron, a potentially life-saving drug that is allegedly being withheld by health officials from Israelis suffering from mild and moderate COVID-19 symptoms.
Following a report on Ynet earlier this month that showed the drug was not offered to most patients because of its high cost, the ministry said the Regeneron will be given by HMOs after it was found effective in preventing deterioration and often death as a result of the virus.
Regeneron, an FDA-approved treatment with a proven track record in the United States to lower the number of hospitalizations, is a cocktail of two monoclonal antibodies and was recommended by U.S. coronavirus czar Anthony Fauci last month.
"We want people out there, including physicians as well as potential patients, to realize the advantage of this very effective way of treating early infection," Dr. Fauci said. "Clinical trials have demonstrated that early treatment with anti-SARS-CoV-2 monoclonal antibodies can reduce the risk of COVID-19 hospitalization or death by 70 [percent] to 85 percent."
Thousands of doses of the drug were purchased and have already been administered to hundreds of COVID patients but was not available to the general public in all hospitals.
3 View gallery
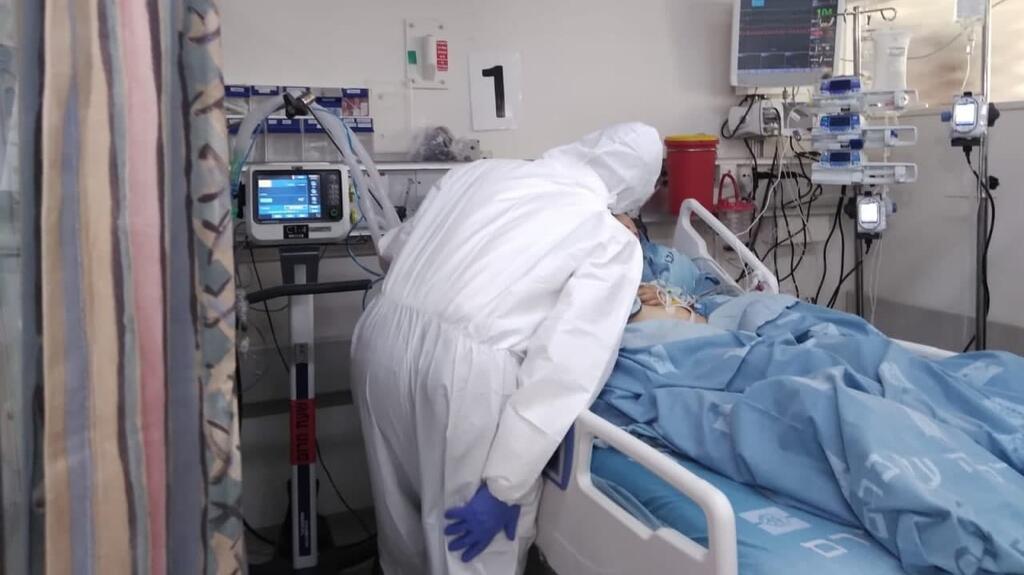

A coronavirus ward at the Hadassah Medical Center in Jerusalem on Monday
(Photo: Hadassah spokesperson)
"Only those who were hospitalized with us were able to receive the drug," one senior official at one of the hospitals said. "Some paid for it from their own pockets. You have to know about it to ask for it," he said.
Other senior health officials said they did not understand why the drug was not available to all those who need it but added that the reason might be bureaucratic struggles and disagreements over who would be responsible for its administration. It is given in an intravenous drip.
The drug is still in its experimental stage and has been given emergency authorization from the FDA. It must be given at the early stages of infection and is estimated to prevent 15% to 20% of daily from deteriorating into a serious illness.
Regeneron can supply immunocompromised patients with the antibodies they need to fight infection from COVID-19 when vaccines do not provide the necessary defense.