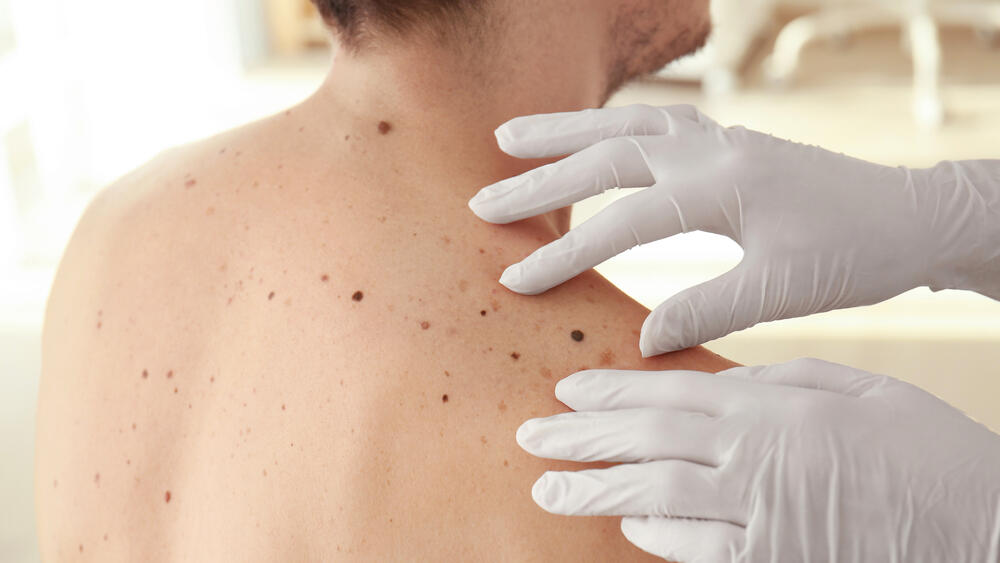
A new study has found that melanoma patients given an experimental mRNA-based cancer vaccine, along with immunotherapy Keytruda, had a 44% lower risk of recurrence or death compared to treatment with Keytruda alone, drugmakers Moderna and Merck revealed at a meeting of the American Association for Cancer Research this week in Orlando, FL.
Other stories:
The company stated that combining the vaccine with the drug may prolong the disease's remission. Dr. Ryan Sullivan, a melanoma specialist who led the study, called it a "potential major breakthrough.”
Moderna and Merkc's investigational melanoma treatment protocol is just one of several combinations of mRNA technology and immune response-boosting drugs whose efficacy is currently being tested out by the scientific community for different types of cancer treatment.
Pfizer subsidiary BioNTech, known primarily for its COVID-19 vaccine, has joined forces with Grifols in developing a similar treatment.
Moderna's new cancer vaccine is personalized according to the biological profile of tumor cells removed from the patient's body during surgery. Based on this profile, the tailored vaccine trains the patient's immune system to recognize and respond to up to 34 distinct proteins called neoantigens, found in the person’s cancer cells but not in healthy ones.
The drug Keytruda, which has been approved for the treatment of melanoma and other types of cancer, belongs to a group of immunotherapies that block a protein called PD-1, which helps cancer cells evade the immune system.
In Merck and Moderna's study, researchers looked into both male and female melanoma patients who had recovered but remained at high risk of relapse. Out of 107 participants who received the experimental treatment protocol, just 22% had recurrences (24 people) within two years.
Out of a control group of 50 patients who had received only Keytruda, cancer had returned in 40 percent of patients (20 people).
Participants in both groups suffered from side effects, mainly fatigue.
The vaccine is currently in the third phase of clinical trials, during which it is being tested on hundreds or thousands of patients in combination with Keytruda. This phase is expected to conclude in three to four years.
Merck is now planning an additional testing phase before seeking FDA approval of the treatment protocol, a process which may take at least four years.
According to Eliav Barr, the company's head of Global Clinical Development and Chief Medical Officer, the development of a personalized vaccine for each patient takes about eight weeks.
He added that since the new mRNA technology allows the body to target dozens of cancer-related mutations at once, unlike previous technologies that only targeted one, it significantly increases the chances of recovery.